Original Post
Date Issued: March 1, 2022
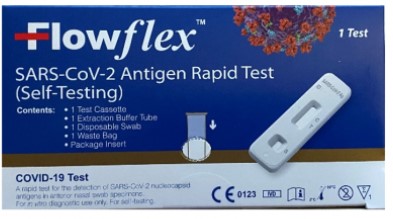
The U.S. Food and Drug Administration (FDA) is warning people not to use certain ACON Laboratories COVID-19 tests. People should not use the ACON Laboratories test named “Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing)” that is packaged in a dark blue box (see image below). This test has not been authorized, cleared, or approved by the FDA for distribution or use in the United States. The FDA is concerned about the risk of false results when using this unauthorized test.
The FDA has not received reports of injuries, adverse health consequences, or death associated with use of the unauthorized ACON Laboratories Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing).
Recommendations
If you have an ACON Flowflex COVID-19 test, compare the packaging to the image above. Do not use the ACON Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) in the dark blue packaging, as shown above.
- Test users and caregivers: Talk to your health care provider if you think you were tested with the ACON Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) and you have concerns about your test results.
- Health care providers and testing program organizers: If an antigen test was performed less than two weeks ago using the ACON Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing), consider retesting your patients using an FDA authorized SARS-CoV-2 diagnostic test if you suspect an inaccurate result. If testing was performed more than two weeks ago and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.
- Report any problems you experience with the Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) to the FDA, including suspected false results. See Reporting Problems with Your Test.
Test Description
- The unauthorized Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing) uses a nasal swab sample to detect proteins, called antigens, from SARS-CoV-2, the virus that causes COVID-19. The unauthorized test has a dark blue box with while lettering and symbols in the lower right corner of the box, including the letters “CE.”
- The ACON Laboratories Flowflex COVID-19 Antigen Home Test, authorized by the FDA on October 4, 2021, is not the subject of this Safety Communication and can continue to be used.
Risks of False Test Results
- A false-negative antigen test result means that the test says the person does not have COVID-19 but they actually do have COVID-19. A false-negative result may lead to delayed diagnosis or inappropriate treatment of SARS-CoV-2, which may cause people harm including serious illness and death. False-negative results can also lead to further spread of the SARS-CoV-2 virus, including when people are housed together in health care, long-term care, and other facilities based on these false test results. When false negative test results are received, actions to limit exposure to an infected person might not be taken, such as isolating people, limiting contact with family and friends, or limiting access to places of employment.
- A false-positive antigen test result means that the test says the person has COVID-19 but they are actually do not have COVID-19. A false-positive result may lead to a delay in both the correct diagnosis and appropriate treatment for the actual cause of a person’s illness, which could be another life-threatening disease that is not COVID-19. False-positive results could also lead to further spread of the SARS-CoV-2 virus when presumed positive people are housed together.
FDA Actions
The FDA regularly monitors the marketing of unauthorized, unapproved, or uncleared tests, including reports of problems with test performance or results. The FDA is working with ACON Laboratories, Inc. to resolve this safety issue.
ACON Laboratories, Inc. has initiated a recall for all unauthorized Flowflex SARS-CoV-2 Antigen Rapid Tests (Self-Testing) tests that were distributed in the U.S.
The FDA will continue to keep the public informed of significant new information.
Reporting Problems with Your Test
If you think you had a problem with a SARS-CoV-2 test, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
Health care personnel employed by facilities that are subject to the FDA’s user facility reporting requirements should follow the reporting procedures established by their facilities.
Questions?
If you have questions about this Safety Communication, email the Division of Industry and Consumer Education (DICE) at [email protected] or call 800-638-2041 or 301-796-7100.
