Do not use recalled Similac, Alimentum, and EleCare powdered infant formulas produced in Sturgis, Michigan.
Original Post

If you use powdered infant formula, be aware certain Similac, Alimentum and EleCare products have been recalled and should not be used.
The U.S. Food and Drug Administration (FDA) is investigating consumer complaints of bacterial infections in five infants who consumed powdered infant formula produced in Abbott Nutrition’s facility in Sturgis, Michigan. All five infants had to be hospitalized and the bacterial infection may have contributed to death in two patients.
Because infant formula is the only source of nutrition for many newborns and infants, the FDA understands and shares the concerns parents and caregivers may have.
Here’s information to help you as we continue our investigation.
What powdered infant formula products have been recalled?
Abbott Nutrition has recalled certain powdered infant formula products produced at its Sturgis, Michigan facility. Products from that facility can be found across the U.S. and some were exported to other countries. Here’s how you can tell if you have any of those products.
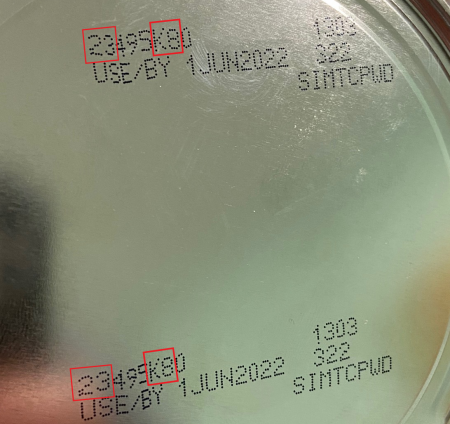
The FDA recommends consumers look at the lot code, a multidigit number on the bottom of a container of Similac, Alimentum and EleCare powdered infant formula and do not use if:
- the first two digits of the code are 22 through 37; and
- the code on the container contains K8, SH or Z2; and
- the expiration date is 4-1-2022 (APR 2022) or later.
In addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code of 27032K80 (can) / 27032K800 (case).
You can also enter your product lot code on the company’s website to check if it is part of the recall. Please see the images below for a closer look at the identifying information.
Powdered Abbott products that don’t have the code and expiration noted above are not included in the recall. Liquid formula products are not subject to the recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.


What infections have been reported and what symptoms should I look for?
Four cases involve Cronobacter sakazakii, and one involves Salmonella Newport infection.
- Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine). Cronobacter infections are rare but are especially high risk for newborns.
- Salmonella are a group of bacteria that can cause gastrointestinal illness and fever called salmonellosis.
- Symptoms related to Cronobacter and Salmonella infection include: poor feeding, irritability, temperature changes, jaundice, grunting breaths, abnormal body movements, lethargy, rash or blood in the urine or stool.
- If your infant is experiencing symptoms related to Cronobacter or Salmonella infection, contact your child’s health care provider to report his or her symptoms and receive immediate care.
When and where were the illnesses?
Illnesses occurred in Minnesota, Ohio, and Texas between September 16, 2021 and January 4, 2022.
I’m having a hard time finding formula. What is the FDA doing to help?
We are aware the recall has created new concerns about the availability of certain types of infant formula, particularly given the overall strains on supply chains experienced during the COVID-19 pandemic.
The FDA is working with Abbott Nutrition to better assess the impacts of the recall and understand the production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott on safe resumption of production at the Sturgis, Michigan facility. As Abbott Nutrition was initiating its recall, the FDA intensified outreach to other infant formula manufacturers to inquire about their capacity and potential impacts. We will continue discussion with Abbott Nutrition and other infant formula manufacturers and consider all tools available to support the supply of infant formula products.
Are homemade formulas an alternative?
No. The FDA advises parents and caregivers not to make or feed homemade formula to infants. Homemade infant formula recipes have not been evaluated by the FDA and may lack nutrients vital to an infant’s growth.
What else should I know?
Parents and caregivers also should never dilute infant formula. Consumers also should avoid buying formula online that comes from outside the U.S., as it has the potential to be counterfeit.
If your regular formula is not available, contact your child’s health care provider for recommendations on changing feeding practices.
If you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance.
Additional Resources:
- Infant Formula: Safety Do’s and Don’ts, FDA Consumer Update
- FDA Investigation of Cronobacter and Salmonella Complaints: Powdered Infant Formula (February 2022), FDA webpage
- Cronobacter Infection and Infants, CDC webpage
